Source of the photo
http://www.aluminum-production.com/process_basics.html
Author of the description
Ujaczki Eva
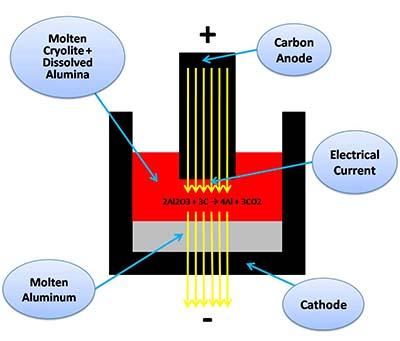
The electrolyte a molten salts called Cryolite (Na3AlF6) used in the Hall–Héroult electrochemical smelting process to dissolve alumina [aluminum oxide (Al2O3)].
"The Carbon anodes are immersed into the electrolyte (usually referred as the "bath") carrying electrical current which then flows into the molten cryolite containing dissolved alumina. As a result, the chemical bond between aluminum and oxygen in the alumina is broken, the aluminum is deposited in the bottom of the cell, where a molten aluminum deposit is found, while the oxygen reacts with the carbon of the anodes producing carbon dioxide (CO2) bubbles."

Source of description
http://www.aluminum-production.com/process_basics.html